1.2 Properties of state
Substance is usually encountered in one of the
three main states: gaseous, liquid, or solid[1].
It is clear that under various conditions one and the same body or substance
can exist in different states. In given permanent conditions, the considered
substance will always be in one and the same state. For instance, at
atmospheric pressure and a temperature of 400 °C water will exist only in the vapor
state but never as a liquid or a solid.
In order to determine the concrete physical
conditions under which a substance is considered and, thereby, determine
unambiguously the state of this substance, convenient characteristics of the
state of substance are introduced, the so-called parameters,
or properties,
of state.
We distinguish intensive and extensive properties
of a substance. Intensive properties
(pressure, temperature and some other) do not depend on the mass of substance
in a system. The properties which depend on the mass of substance in a system
are referred to as extensive. An
example of extensive properties is volume, which changes under given conditions
in proportion to the mass of substance: the volume of 10 kg of substance is 10
times greater than the volume of 1 kg of the same substance.
The specific extensive properties, i.e. properties
reduced to unit mass of substance, acquire the meaning of intensive properties.
So, for instance, specific volume, specific heat capacity, etc., can be
considered as intensive properties. The intensive properties, determining the
state of a body or of a group of bodies - the thermodynamic system - are
referred to as the thermodynamic properties of
the state of a body (system).
The most convenient and, therefore, the most widely
used properties of state are the absolute temperature, the absolute pressure
and the specific volume (or density) of a body.
One of the most important properties of state is absolute
temperature. Temperature characterizes
the thermal state of a body. It is common knowledge that heat can flow
spontaneously only from bodies at a higher temperature to bodies at a lower
temperature. Thus, the temperature of bodies determines the direction of the
possible spontaneous heat exchange between these bodies[2].
Temperature is measured, for instance, with the aid
of thermometers. Since the physical properties of substances depend to a greater
or lesser extent on temperature, we can use as a thermometer an instrument
based on accurate and easily reproducible measurements of such properties.
Each instrument used for
measuring temperature must be calibrated in accordance with a commonly accepted
temperature scale. The relationships between the presently used Celsius, Fahrenheit,
Reaumur and Rankine temperature scales are given in Table 1.1; the most
commonly used is the Centigrade (Celsius) temperature scale in which the
temperature interval between two fixed points, the ice melting point (0°C) and
the boiling point of water at atmospheric pressure (100 °C) is divided into one
hundred equal intervals (degrees).
Table 1.1 Relations between
Celsius, Rankine, Fahrenheit and Reaumur temperature scales
|
Scale |
Celsius, oC |
Rankine, oRa |
Fahrenheit, oF |
Reaumur, oR |
|
Celsius, oC |
- |
|
|
|
|
Rankine, oRa |
|
- |
|
|
|
Fahrenheit, oF |
|
|
- |
|
|
Reaumur, oR |
|
|
|
- |
Of essential importance in thermodynamics is the
so-called thermodynamic, or
absolute, temperature scale, which
shall be considered below, in Chapter 3.
The other important property of state, absolute
pressure,[3] is
defined as the force per unit area exerted by the substance on its
surroundings. Pressure is measured in different units: the pascal (Pa)[4],
the bar, the so-called technical atmosphere or simply atmosphere (1 kgf/cm2),
in mm of Hg or H2O. The relations between different units of
pressure are given in Table 1.2.
Table 1.2 Relations between different units of pressure
|
Unit |
Pa |
bar |
kgf/cm2 |
mm
Hg |
mm H2O |
|
1 Pa |
1 |
10-5 |
1.02×10-5 |
7.5024×10-3 |
0.102 |
|
1
bar |
105 |
1 |
1.02 |
7.5024×102 |
1.02×104 |
|
1
kgf/cm2 |
9.8067×104 |
0.98067 |
1 |
735 |
104 |
|
1 mm
Hg |
133 |
1.33×10-3 |
1.36×103 |
1 |
13.6 |
|
1 mm
H2O |
9.8067 |
9.8067×10-5 |
10-4 |
7.35×10-2 |
1 |
The specific volume of
substance is the volume occupied by unit mass of a substance. The specific
volume v is related to the mass of a
body G and its volume V
as follows:
![]() (1.1)
(1.1)
The specific volume of
substance is usually measured in m3/kg or cm3/g.
Density
![]() (1.2)
(1.2)
is usually measured in kg/m3
or g/cm3.
The concept of the specific weight (gravity) of
substance will sometimes be encountered in the text. By specific weight we mean
the weight of substance per unit volume. In accordance with Newton's Second
Law, the density and specific weight of a substance are related by the
following equation:
![]() (1.3)
(1.3)
where g
is the acceleration of gravity.
In the absence of external forces, the state of
pure substance is determined unambiguously if two intensive independent
properties of state are given. Any other property is a single-valued function
of these properties of state. If we consider, for instance, water vapor at a temperature
of 250°C and a pressure equal to 98 kPa (10 kgf/cm2), there can be
only one value for the specific volume of such vapor, v
= 0.2375 m3/kg. Thus, the specific volume
of a given substance is determined unambiguously by the pressure p
and temperature T, i.e.
![]() (1.4)
(1.4)
Since in determining the state of a substance all
state properties "are equal in rights", the temperature of the
substance will be unambiguously determined from the relation
![]() (1.5)
(1.5)
and the pressure from the
relation
![]() (1.6)
(1.6)
Thus, any three properties
of state (for instance, p, v or T) of a pure substance are
interrelated unambiguously. The equation relating the three properties is
referred to as the equation of state of a given substance. The relations (1.4) to (1.6)
can be presented as the equation of state in the following form:
![]() (1.7)
(1.7)
For each substance the functional relation between p, v, and
T (or the values of constants
present in this relation) is individual in nature; hence, the thermodynamic
properties are described by an equation of state which is proper for each
substance.
The relation between the properties of state can be
represented by a system of coordinates p,
v, T in the form of a so-called thermodynamic
surface, illustrated in Fig. 1.1a.
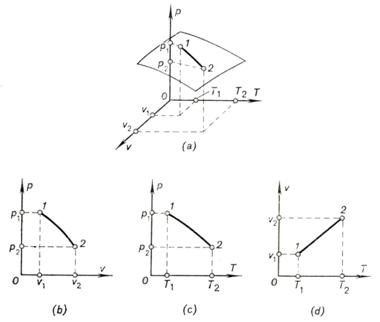
Fig. 1.1
However, the representation of the states of a
system (substance) and of the processes developing in the system in space (or
three-dimensional) coordinates involves a number of difficulties. That is why
rectangular coordinates are usually used, with any two (of the three)
properties of state taken for abscissa and ordinate. The magnitude of the third
property is then determined for each pair of given properties from the
equation of state or by experiment.
Coordinate systems of this kind are usually
referred to as the diagrams of state, or
diagrams of properties. The
diagrams finding the widest application in engineering thermodynamics are those
with coordinates p and
v (the p-v
diagram Fig. 1.1b),
p and T
(the p-T diagram,
Fig. 1.1c), and v and
T (the v-T
diagram, Fig. 1.1d). Below we shall get familiar with a number of other diagrams of
properties, or state diagrams.