1.5 Concept of mixtures. Mixtures of ideal gases
Pure
substances and mixtures
In thermodynamics the concepts of a pure substance
and a mixture (solution) is used. By a pure substance we
mean a substance all molecules of which are similar, or a substance which does
not change chemically. A mixture, consisting
of several pure substances, is referred to as a solution.
Examples of pure substances are water, ethyl
alcohol, nitrogen, ammonia, sodium chloride, and iron. Examples of mixtures are
air, consisting of nitrogen, oxygen and a number of other gases, aqueous
ammonia solutions, aqueous solutions of ethyl alcohol, various metal alloys.
The pure substances making up a mixture are called components
or constituents.
Determining
the composition of a mixture
One of the most important characteristics of a
mixture is its composition. The composition of a mixture is usually determined
by finding the mass and molar fractions of the individual mixture components.
Let us consider a mixture consisting of G1 kg
of the first component, G2 kg
of the second component, G3 kg
of the third component, etc. The total mass of a mixture consisting of n
components will be
![]() (1.34)
(1.34)
The mass fraction of each component is defined as
the ratio of the mass of the given component to the mass of the entire mixture
![]() (1.35)
(1.35)
It follows from Eqs. (1.34) and (1.35) that
![]() (1.36)
(1.36)
Consequently, the mass fraction can be determined
by the fraction of the given component contained in 1 kg of the mixture.
For a mixture consisting of two components
(referred to as a binary mixture)
![]()
Therefore,
the composition of the mixture is known if the mass fraction of one of the
components is given. In a binary mixture, the mass fraction of the second
component is usually denoted by c,
and it is then evident that the mass fraction of the second component is (1 - c). Thus,
for a binary mixture
![]()
![]() (1.37)
(1.37)
![]()
It is sometimes more convenient to determine the
composition of a mixture by means of molar fractions. By a molar fraction of a
mixture component is meant the ratio of the number of moles of the component
considered to the total number of moles of the mixture.
Let a mixture contain M1 moles
of the first component, M2 moles
of the second component, M3 moles
of the third component, etc. The number of moles of the mixture is equal to
![]() (1.38)
(1.38)
and
the molar fraction of the ith
component
![]() (1.39)
(1.39)
In accordance with Eqs. (1.38) and (1.39),
![]() (1.40)
(1.40)
Thus, the molar fraction of a given component in a
mixture can be defined as the number of moles (the fraction of a mole of this
component) in one mole of the mixture:
![]()
Just as for mass fractions, the letter N
is used below to denote the molar fraction of the
second component in a binary mixture, and the molar fraction of the first
component is denoted by (1 - N). Then,
![]()
![]() (1.41)
(1.41)
The relation existing
between the mass and molar fractions permits, if necessary, to express one
fraction in terms of the other. In order to find this relation, let us consider
a mixture with an arbitrary number of components. If the molecular masses of the
first, second, third, etc., components are respectively denoted by µ1,
µ2, µ3, . . ., µn, then the mass fraction of the ith
component, ci, can be expressed in terms of the molar fractions in the following way:
 (1.42)
(1.42)
If the mass fractions of the components of a given
mixture are known, the expression which can be used to determine the molar
fraction Ni of
any ith component of the
mixture takes the form:
 (1.43)
(1.43)
For a binary mixture, from Eqs. (1.42) and (1.43)
it follows that for the first component
![]()
and
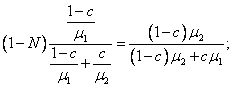 (1.44)
(1.44)
for the second component
![]()
and
 (1.45)
(1.45)
Mixtures
of ideal gases. Dalton's law
Mixtures of various gases, the so-called gas
mixtures, are considered as special cases of mixtures (solutions). It is of
great interest to examine a gas mixture each component of which considered as
an ideal gas. The idea of considering mixture components as ideal gases happens
to be a good approximation for quite a number of gas mixtures at low
pressures. Of such gas mixtures, air is of the greatest practical importance.
The basic law determining
the behavior of a gas mixture is Dalton's law: Each
individual gas behaves in a gas mixture as though it alone occupies the volume
of the mixture at the mixture temperature.
In other words, each individual component of a gas
mixture is under the pressure which it would exert if it alone occupied the
volume of the mixture. This pressure is called the partial
pressure of the given gas, and the
partial pressure of each mixture component is respectively denoted by p1, p2, p3,
. . . . . ., pn. At
first glance it may seem strange that it is indifferent for the given gas
component whether there are any other gases in the volume or it alone occupies
the space. However, there is nothing strange in the phenomenon, since we are
dealing with ideal gases and, as mentioned above, the molecules of an ideal gas are,
by definition, material points devoid of volume and not interacting with one
another in any way, except by collision. The higher the pressure in a mixture,
i.e. the more the states of the gases deviate from the ideal state, the more
the behavior of the gas mixture is observed to deviate from that prescribed by
Dalton's law. Dalton's law can also be stated as follows:
The sum of the partial pressures of
the ideal gases which are components of a gas mixture
is equal to the total pressure of the mixture of gases:
![]() (1.46)
(1.46)
Dalton's law finds wide application in describing
various mixtures of gases, and it will be repeatedly used in the book.
Composition
of an ideal gas mixture. Volume fractions
If a component of an ideal
gas mixture is not at its partial pressure at the temperature of the mixture
but the total pressure of the mixture is exerted on it, its volume is Vi, which is referred to as the partial volume of the ith gas component. The ratio between the partial volume
and the volume of the mixture is called the volume fraction of the given component:
![]() (1.47)
(1.47)
The
partial volume is determined from Boyle's law
![]()
where pmix and Vmix are the pressure and volume of the mixture;
![]()
whence
![]()
Since
![]()
we
get
![]() or
or ![]() (1.48)
(1.48)
Thus, the total volume of a mixture of gases is
equal to the sum of the partial volumes of its components.
If a gas comprises M1, M2, . . ., Mi moles of different gases,
the volume fraction of the ith
component is equal to:
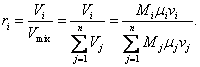 (1.49)
(1.49)
Since for all gaseous components of the gas
mixture, reduced to the same pressure pmix and the same temperature Tmix, the volume of the moles is the same, we have
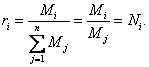 (1.50)
(1.50)
Thus, the volume fraction of the component of a mixture
of ideal gases is equal to the molar fraction of this component.
It follows from this that Eqs. (1.42) and (1.43)
can be presented in the following form:
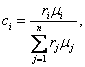 (1.51)
(1.51)
 (1.52)
(1.52)
Apparent
or average molecular (molar) mass of a mixture
In calculating mixtures of ideal gases, it is
convenient to make use of the so-called apparent or average molecular (molar)
mass of the mixture, which is the ratio of the mass of the mixture to the total
number of moles of all mixture components:
![]() (1.53)
(1.53)
Taking into account Eqs. (1.30), (1.35) and (1.38),
we find that
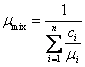 (1.54)
(1.54)
and
![]() (1.55)
(1.55)
Gas
constant of a mixture
For the ith component of a mixture of
ideal gases with a volume Vmix Clapeyron's equation will obviously take the form
![]()
Writing this equation for all components of the
mixture and summing the left-hand and right-hand sides, we get:
![]() (1.56)
(1.56)
In accordance with Dalton's
law, ![]() ,whence
,whence
![]() (1.57)
(1.57)
On the other hand, for a mixture of ideal gases
Clapeyron's equation can obviously take the following form:
![]() (1.58)
(1.58)
where Rmix is the gas constant of the mixture.
It follows from Eqs. (1.57) and (1.58) that
 (1.59)
(1.59)
Since
![]()
from Eq. (1.59) we get
![]() (1.60)
(1.60)
whence, with account taken
of Eq. (1.57), we should get:
![]() (1.61)
(1.61)
Substituting µmlx from Eq. (1.55), we
obtain:
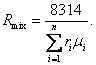 (1.62)
(1.62)
Determining
partial pressures
Since piVmix = pmixVi and Vi/Vmix = ri,
![]()
and
![]() (1.63)
(1.63)