1.6 Concept of heat capacity
The heat capacity of
a body is defined as the quantity of heat required to raise the temperature of
the body by one degree.
It follows from this definition that the heat
capacity of substance is an extensive property of a body. Indeed, the heat
capacity of a given body is the greater, the greater the amount of substance
contained in this body. For instance, the heat capacity of 10 kg of water is
five times greater than the heat capacity of 2 kg of water.
The heat capacity of a unit mass of substance is
given the name of specific heat. In
accordance with what was said in Sec. 1.2, it is clear that specific heat is an
intensive property of a substance, i.e. its magnitude does not depend on the
amount of substance in the system.
Average heat capacity and
true heat capacity
Heat capacity will be denoted by c[1]. From
the definition of heat capacity, it follows that
![]() (1.64)
(1.64)
where
t1 is
the initial temperature, t2 the
final temperature, and q12 the
heat added to unit mass of substance while heating it from temperature t1 to
temperature t2.
Heat capacity is not a constant quantity. It
changes with temperature, the dependence being quite substantial in a number of
cases. Therefore, the heat capacity determined from formula (1.64) for the
temperature interval t2 - t1 is
called the average heat capacity[2],
as distinguished from the so-called true heat capacity which
is defined as the derivative of the quantity of heat added to the body with
respect to the temperature of this body:
![]() (1.65)
(1.65)
whence
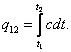 (1.66)
(1.66)
If we plot the amount of
heat added to the body versus temperature (Fig. 1.5), it is clear that due to
the variation of heat capacity the dependence will be represented by the curve q = f (t) and not by a straight line. In accordance with Eq. (1.66), on the curve q = f (t) the average heat capacity can be considered to be
equal to the slope of the secant passing through points 1 and 2, i.e. to tan β, and the true heat capacities in the states 1 and 2; to tan α1 and tan α2,
respectively.
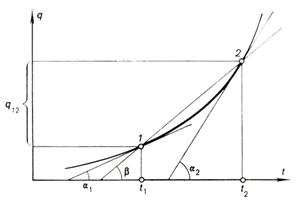
Fig. 1.5
Knowing the dependence of the true capacity on
temperature, we can easily determine the average heat capacity in a given
temperature interval:
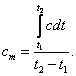 (1.67)
(1.67)
Mass, molar and volume heat
capacities
Mass heat capacity, c, is defined as the amount of heat required to raise the temperature of
unit mass of substance (usually 1 kg or 1 g) by 1 °C.
Molar heat capacity, μc, is the name given to the heat capacity reduced to
one mole (or kilomole) of substance.
The volume heat capacity, C, is defined as the heat capacity reduced to unit volume of substance
(usually to 1 m3).
It is obvious that
![]() (1.68)
(1.68)
In practice, use is made of the mass heat capacity,
which will be referred to below as heat capacity.
Dependence of heat capacity
on process
Depending on the nature of
the process of adding heat, the amount of heat to be added to a body so as to raise
its temperature by 1 degree will differ. When speaking of heat capacity,
therefore, it is necessary to specify which particular process of adding heat
to a body is involved.
To put it differently, the
quantity q, present in Eq. (1.65), depends not only on the temperature interval, but
also on the kind of the process of adding heat. In Eq. (1.65), therefore, the
amount of added heat q must be given a subscript characterizing the kind
of process involved,
![]() (1.69)
(1.69)
where
the subscript x indicates
the property which is preserved constant in the given process.
In practice, the heat capacities of the isobaric (x = p = const) and isochoric (x =
v = const)
processes are usually employed. These heat capacities are referred to as the
isobaric and isochoric heat capacities and they are denoted by cp (heat
capacity at constant pressure) and cv (heat
capacity at constant volume).