2.1 Heat. Joule's experiment. Equivalence of heat and work
Heat is one of the most essential concepts
of thermodynamics. By its nature the concept of heat is very close to the
concept of work. Both heat and work are forms of transfer of energy. There is,
therefore, no sense in saying that a body stores some heat or work. We can only
state that a certain amount of heat or work has been imparted to, or taken away
from, a body.
The difference between heat and work
consists in that they are different forms of transfer of energy. Heat is such a
form of transfer of energy which is associated either with direct contact
between bodies (heat conductivity, convection) or with radiant transfer of
energy. Work is regarded as another mechanism of energy transfer. Mechanical
work is always associated with a change in the volume of the body involved.
It is customary to assume that addition of
heat involves a rise in the temperature of the body, determined by the energy
of the microparticles constituting the body, which is often the case. But, as
it will be seen later on, it happens that not withstanding the addition of heat
to a body its temperature decreases. All depends on the balance of the energy
transferred to the body and removed from it. In the special but most widespread
case the change in the temperature of a body is determined by the relation
between the amounts of heat and work imparted to the body and removed from it.
In the course of the development of science
the ideas about heat varied. From the lime of Aristotle the predominant idea of
heat consisted in that heat was assumed to be one of the 'primary
qualities" of matter, with this "primary quality" being inherent
to every body to a different degree. As long ago as the seventeenth century, it
was evident that
in the works of Descartes and Bacon attempts were made to relate the idea of
heat with the motion of particles of which bodies consist. In the eighteenth
century, in connection with the wide development of calorimetry, a new
scientific idea appeared of the so-called caloric or phlogiston, an inviscid
(non-viscous) and invisible liquid passing from bodies at a higher temperature
to bodies at a lower temperature when they come or are brought into contact;
the increase in the temperature of a body was thought to be due to the increase
in the content of the caloric or phlogiston in the body. It ought to be
mentioned that as long ago as 1760 the famed Russian scientist M. V. Lomonosov
rejected the theory of the phlogiston, postulating the idea of heat as a form
of motion of particles.
At the end
of the eighteenth centuries works appeared refuting the theory of the
phlogiston. One of the first works in this field involved an experiment
conducted by the English physicist Benjamin Thompson (Count Rumford) in 1798.
During the experiment a blunt cutter was pressed to the internal surface of a
barrel of a gun rotating about its axis. Rumford discovered that resulting from
the friction between the cutter and barrel, the temperature of the barrel
increased, due to the liberation, or release, of heat. It was also found that
in the course of the experiment, heat was liberated as long as the barrel
rotated. Analyzing the results, Rumford concluded that if an insulated body or
a system of bodies is capable of an unlimited liberation of heat, then the heat
is not a material substance and only motion is able to ensure a continuous
"excitation and propagation of heat in our experiments".
Simultaneously with Rumford s work, the experiments of another English
scientist, Sir Humphry Davy, dealt a hard blow on the theory of the phlogiston.
The scientist demonstrated that it is possible to melt two pieces of ice or fat
or wax by simply rubbing them against each other, without bringing them in contact
with a higher-temperature body.
In 1844-1854 the English scientist J. P.
Joule conducted experiments which were destined to play an important role in
science. The objective of Joule's experiment was to establish a relation
between the amount of work spent to bring about the liberation of heat and the
amount of the heat liberated. The layout of Joule's experiment was as follows
(Fig. 2.1). Paddle wheel 2 was submerged in the heat-insulated vessel 1
to the walls of which vanes 3 were fastened, the vanes interfering
with the motion of water due to rotation of the paddle. Rotation was imparted
to the paddle (stirrer) by the falling load 4 of weight G, connected
to the paddle by means of a rope and pulley 5. As the weight falls
through a distance Δh, the work
done by it (and, consequently, by the stirrer) is equal to the decrease in the
potential energy of the weight GΔh. The heat liberated in the
water-filled vessel is calculated from the rise in water temperature, measured
with a thermometer.
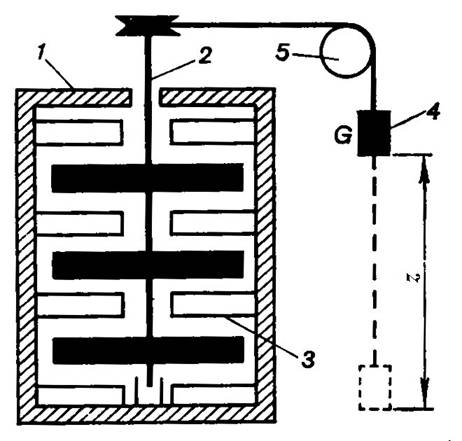
Fig. 2.1
It should be noted that before the nature
of heat was finally established, sufficiently accurate methods of measuring the
amount of heat (calorimetry) were elaborated. The mass of water was measured in
advance, and the absorption of heat by the walls of the vessel, vanes and the
paddle wheel was taken into account. The heat capacities of water and metal
(copper) were known. Based on the results of a series of thoroughly conducted
experiments, Joule discovered a direct proportionality between the spent work L
and the quantity of heat obtained Q:
Q = AL, (2.1)
where A is a proportionality factor.
Joule found that the proportionality
factor A remains the same irrespective of the method of heat production,
type of work, temperature of the body involved, etc.
In other words, Joule established that
when one and the same amount of work is spent, one and the same amount of heat
is liberated. Thus, the amount of liberated heat was shown to be equivalent to
the amount of work spent; it is clear that this relationship is true for the
case when work is accomplished at the expense of heat.
Using the results of these measurements,
Joule calculated the magnitude of A, which is known as the thermal
equivalent of work, and of J,
referred to as the mechanical equivalent of heat:
A = 0.002345 kcal/(kgf·m)
and
J = 427 kgf·m/kcal.
It is obvious that
J = 1/A.
Later on the values of A and J, obtained by Joule, were somewhat
improved; in accordance with the results obtained after very accurate
up-to-date measurements,
J = 426.935 kgf·m/kcal.
As it was already mentioned, sufficiently
accurate methods of measuring heat (calorimetry), based on using the notions of
temperature and heat capacity of a body, had been elaborated as far back as the
eighteenth century, i.e. long before the nature of heat was finally elucidated.
In its time the most common unit of heat was the calorie, which was
defined as the amount of heat required to raise the temperature of 1 g of water
by l oC. However, it was found later that the heat capacity of water
changes somewhat with temperature. Therefore, at different temperatures
different amounts of heat are required to raise the temperature of 1 g of water
by 1°C. In this connection it became necessary to define the calorie with
a greater accuracy, and the so-called 15° calorie was introduced,
defined as the amount of heat required to raise the temperature of water from
14.5 °C to 15.5 °C. At the present time work and heat are measured in different
units; the relations between them are given in Table 2.1. The units most widely
spread are the joule and the international calorie (4.1868 J = 1 cal).
Table
2.1 Relations between different units of work and heat
|
Unit |
J |
erg |
kgf-m |
kcal |
kW-h |
|
1 J |
1 |
107 |
0.101972 |
2.38846×10-4 |
2.7778×10-7 |
|
1 erg |
10-7 |
1 |
10.1972×10-9 |
23.8846×10-12 |
27.778×10-15 |
|
1 kgf-m |
9.80665 |
98.0665×106 |
1 |
2.34228×10-3 |
2.72407×10-6 |
|
1 kcal |
4186.8 |
41.868×109 |
426.935 |
1 |
1.163×10-3 |
|
1 kW-h |
3.6×106 |
36×1012 |
367098 |
859.845 |
1 |
Shortly after Joule's experiments, the
molecular-kinetic theory was elaborated, according to which heat is the energy
of the chaotic thermal motion of the microparticles of a body.
From here on, to simplify notation the
thermal equivalent of work A and the mechanical equivalent of heat J will not be used in the thermodynamic
equations, heat and work will be measured in the same units.