2.6 Mathematical statement of the first law of
thermodynamics for processes of flow
Up till now we were considering only
systems in which the substance did not move (as a whole) in space. It should be
stressed, however, that the first law of thermodynamics is of a general nature
and is true for any system, both fixed and moving.
Let us consider the flow of a liquid or
gas in a conduit of arbitrary form. In considering the flow, one more factor
should be taken into account, the kinetic energy of flow
![]() (2.55)
(2.55)
where G is the mass of some fixed quantity of
substance in the flow, and w is the flow velocity.
If the velocity of flow changes along the
conduit between two sections (let us denote them 1 and 2, Fig. 2.6), the kinetic energy of
the flow changes by
![]()
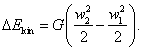 (2.56)
(2.56)
Let us consider the equation of the first
law of thermodynamics (2.15a):
Q1-2 = (U2 – U1)
+ L1-2.
Here the subscripts "1" and "2"
are referred to the two sections of the flow (Fig. 2.6). Let us analyze the
components of the quantity L1-2,
present in this equation, as applied to the flow; for this purpose let us
investigate what kinds of work are performed by the flow of gas (or liquid).
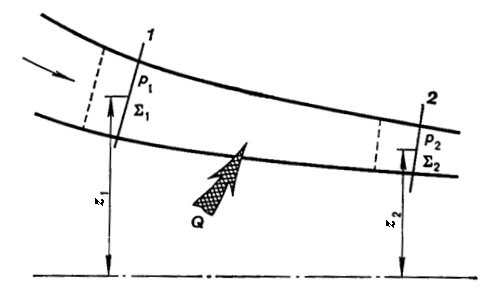
Fig. 2.6
So, consider the flow in a conduit of
arbitrary form (Fig. 2.6). A certain amount of heat (Q12 can be added to the flow between the sections 1 and
2.
Denote the cross-sectional area 1 of
the conduit by Σ1, and that of section 2 by Σ2.
The pressures under which the substance flows through the sections 1 and
2 are denoted, respectively, by p1
and p2.
The amount of substance passing through a
section of the conduit per unit time (the mass flow rate) denote by G. It
does not matter which of the two sections are chosen to measure the mass flow
rate G; in accordance with the
principle of continuity of flow, known from hydraulics, the mass flow rate of
stationary flow is the same for any section of the conduit involved (G = const).
Let us determine the work performed by the
flow.
In order to introduce a portion of the gas
or liquid of mass G into the considered length of the conduit, through
section 1 per unit time, it is necessary to spend a certain amount of work
to displace a similar portion of the gas from the considered length of the
passage, freeing, thereby, the space for the newly introduced portion of gas.
Let us denote by x1
the path covered by the portion of gas considered per unit time, as it
passes through section Σ1. For the sake of clarity, the section
of this portion of gas can be visualized as moved by a piston without friction
(inasmuch as in the general case the section of the conduit is variable, the
fictitious piston should be of a variable cross-section; this assumption in no
way effects the results obtained). In order to displace the piston by a
distance x1, an amount of
work must be performed equal to the product of the force acting upon the piston
(this force is equal to the product of gas pressure p1 by the cross-sectional
area of the piston Σ1) by the length of piston travel per unit
time:
L(1) = p1 Σ1x1.
Denote
V1 = x1 Σ1.
where V1 is the volume of the gas passing into the part of
the conduit considered per unit time. It is clear that
V1 = v1G,
where G is the mass flow rate through passage,
and v1 the
specific volume of gas in section 1.
Thus,
L(1) = - p1 v1G. (2.57)
It should be noted that L(1) is the work done on the
flow and, therefore, the quantity L(1) is negative [the "minus" in (2.57)].
The work done by the piston moving through
section 2 is calculated in a similar manner
L(2) = p2 V2.
In accordance with the principle of
continuity of flow formulated above, the mass rate of gas flow through section 2
is the same as through section 1; therefore,
V2 = v2G
and
L(2) = p2V2G. (2.58)
where L(2) is the work performed by the flow (piston 2) emerging from section 2. Consequently,
this work is assumed to be positive.
It follows from Eqs. (2.57) and (2.58)
that as the gas flows in a conduit from the arbitrarily chosen section 1 to
section 2 at a mass flow rate equal to G, the work performed per unit time is equal to the algebraic sum
of the work L(2) done
by piston 2, and the work L(1) done on piston 1 (this work is called
the work of displacement or transport work),
Ldis = L(1) +
L(2),
whence, in accordance with (2.57) and (2.58), we have
Ldis = (p2v2 - p1v1)
G. (2.59)
The work of displacement is the first component
of the work done by the flow.
Further, if the flow velocity in section 2, w2, differs
from the flow velocity
in section 1, w1,
then to change the kinetic energy of the flow, determined by Eq. (2.56),
energy must be added (or removed):
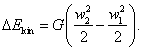
This is the second component of the work
performed by the flow.
If the sections 1 and 2 are
arranged at a different height (z1
and z2, respectively),
work must be expended to elevate the considered portion of the gas from height z1 to height z2. This work is equal
to the change in the
potential energy of the portion of gas of mass G; in accordance with Eq. (2.12),
Lpot = Gg(z2 – z1).
This is the third component of the work
done by the flow. In the general case the flow is also capable of performing
other kinds of work as it passes from section 1 to section 2 of the
conduit, for instance, rotate the wheel of a turbine or, if the flow of a
conducting liquid in a cross magnetic field is involved, deliver electric power
into an external circuit due to the magneto hydrodynamic effect, etc. All these
kinds of work, referred to as mechanical work, we denote by Lmech. Mechanical work can
not only be removed from a flow but also added to it: a flow can be delivered
by a centrifugal pump, or handled by an electromagnetic pump, etc.
The mechanical work is the fourth
component of the work performed by the flow.
Finally, the fifth component of the work
of flow is the work spent to overcome skin friction between the flow and the
walls of the conduit. Denote this kind of work by Lfr.
It follows that the work accomplished by a
flow of gas (fluid or liquid) can be expressed in the general case by the following
formula:
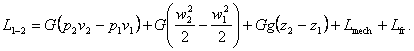 (2.60)
(2.60)
Substituting the relationship for L12 in the equation of the
first law of thermodynamics (2.15a), we get:
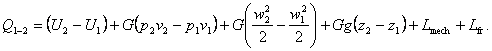 (2.61)
(2.61)
Dividing both sides of Eq. (2.61) by G, we obtain a similar relationship
for a unit mass of flow
(i.e. expressed in specific quantities):
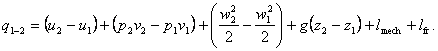 (2.62)
(2.62)
In the differential form this equation
becomes
![]() (2.63)
(2.63)
Taking into account that
h
= u +
pv,
we get:
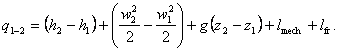 (2.64)
(2.64)
and
dq
= dh + wdw + gdz + dlmech+ dlfr. (2.65)
Equations (2.64) and (2.65) are the mathematical formulation of the
first law of thermodynamics for a flow process.
Let us compare now the differential equation of the first law of
thermodynamics, written in the most general form for an arbitrary system, with
the private case of this equation for a flow process. The first of these
equations, Eq. (2.23), is
dq
= du + pdv,
and the
second, Eq. (2.63),
dq
= du +d(pv) + wdw + gdz
+ dlmech+ dlfr.
It should be borne in mind that Eq. (2.23)
is written for the case when the only kind of work performed is expansion work.
In this connection it should be stressed that the mechanical work accomplished
by the flow, lmech
should not be confused with the work l*, present in Eq. (2.21), performed by the system on
other, apart from pressure, generalized forces; when the velocity of flow is
equal to zero, no mechanical work is performed, while the magnitude of l* does not depend on the
velocity of the system (for instance, the work spent to overcome the resistance
of surface tension while the surface is increasing).
It should be also stressed that in the
case of frictional flow the work of the flow spent to overcome friction turns
fully into heat transferred to the flow. Therefore, for the case of frictional
flow, the quantity q in the left-hand side of Eqs. (2.23) and (2.63),
represents the sum of the heat added to the flow from an outside source
(denoted by qext) and the frictional heat qfr:
q
= qext + qfr. (2.66)
Equations (2.23) and (2.63) can be then
presented as follows:
dqext + dqfr = du +pdv; (2.67)
dqext + dqfr
= du + d(pv) + wdw+gdz + dlmech + dlfr. (2.68)
As
regards Eq. (2.68), inasmuch as
dqfr = dlfr,
the quantities dqfr and dlfr in this equation cancel out and the
equation takes the following form:
dqext = du + d (pv) + wdw + gdz + dlmech (2.68a)
or, which is the same,
dqext = dh+ wdw + gdz + dlmech, (2.68b)
but in Eq. (2.67) dqfr is not cancelled out, since it is only one of the components of expansion work pdv.
In principle, Eqs. (2.23) and (2.63) are
identical. They express analytically the first law of thermodynamics. For this
reason the right-hand sides of these equations can be equated. Then,
pdv = du + d(pv) + wdw+gdz + dlmech
+ dlfr (2.69)
Equation (2.69) shows that the work spent
to displace the flow, d(pv), to change its kinetic energy, wdw, to
change the potential energy of the flow, gdz, to overcome the resistance
to flow offered by frictional forces, dlfr, and the mechanical work, dlmech, are
performed at the expense of the work of expansion done by the gas (or liquid)
in the flow, i.e. pdv. For if a flowing gas expands (i.e. if its specific
volume increases), work must be accomplished, involved in the increase of v; the differential of this
work is always equal to the product pdv.
Inasmuch as
d(pv)
= pdv + vdp,
it follows from Eq. (2.69) that for any
flow the following is always true:
wdw
= - vdp - gdz - dlmech
- dlfr. (2.70)
For the case where the flow does not
perform any mechanical work (dlmech = 0), we obtain
wdw=
- vdp – gdz
– dlfr. (2.71)
When dz = 0, from equation (2.71)
it follows that
wdw
= - vdp – dlfr. (2.72)
Finally, for the case of frictionless flow
wdw
= - vdp. (2.73)
These important relationships will be used
in Chapter 8.