4.1 Basic methods
The combined mathematical expression for the first and second laws of
thermodynamics formulated in the preceding chapter has the following
appearance:
![]() (4.1)
(4.1)
On the basis of this equation with the aid of mathematical methods we
can obtain a number of equations interrelating the various thermodynamic
properties of substances.
As was already mentioned, all thermodynamic quantities can be divided
into two groups, functions of state and functions of a process. The magnitude
of a function of state is determined unambiguously by the properties, or
parameters, of a given state. Hence, to determine the change of a function of
state, we must only know the values of this function at the beginning and end
of the process.
The functions of a process (for instance, work and heat) are determined
by the nature of the process causing a change in the state of a thermodynamic
system.
As was already mentioned in Chapter 2, the characteristic property of
functions of state is that their differential is a total differential. It will
be recalled that in mathematical analysis the name total differential of a
function of several variables, z = f (x, y, w, . . .), is given to
a quantity of the following kind
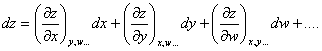 (4.2)
(4.2)
In the great majority of cases, in dealing with pure substances we shall
consider functions of two variables, z = f (x, y), for which
![]() (4.3)
(4.3)
Partial derivatives are taken on condition that the superscripts put
outside the parenthesis are held constant during the differentiation. For
instance, the derivative of pressure in respect to temperature,
![]() can be
calculated for various conditions: on condition of a constant system volume v, on condition that the
system's entropy s is constant, or that the system's enthalpy h is
constant, etc. In each of these cases the derivative, respectively denoted by
can be
calculated for various conditions: on condition of a constant system volume v, on condition that the
system's entropy s is constant, or that the system's enthalpy h is
constant, etc. In each of these cases the derivative, respectively denoted by ![]()
![]()
![]() etc. will have, generally speaking, a
different value. It is known from mathematical analysis that
etc. will have, generally speaking, a
different value. It is known from mathematical analysis that
![]() (4.4)
(4.4)
i.e.
the magnitude of a partial derivative of a higher order does not depend on the
sequence of differentiation.
From this it follows that if the differential of any function z = f (x, y) is presented in the form
![]() (4.5)
(4.5)
and if
it is known that the differential of the function z is a total differential, the following relationship is true:
![]() (4.6)
(4.6)
Equation (4.6) will be used to obtain a number of important differential
equations of thermodynamics.
Equation (4.6) can be used, in particular, to show that the differential
of a function of a process is not a total differential. Consider, for instance,
the expression for the differential of the amount of heat added to a system,
i.e. the mathematical formulation of the first law of thermodynamics (2.23),
![]()
In order to represent q as a function of two variables p and
v, substitute the
relationship
![]()
for the
differential of internal energy. We obtain:
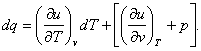 (4.7)
(4.7)
We shall now check whether this relationship meets the condition
formulated by Eq. (4.6). As applied to Eq. (4.7),
![]()
![]()
![]()
![]()
whence
![]() (4.8)
(4.8)
![]() (4.9)
(4.9)
With account taken of condition (4.4) it follows that the condition
(4.6) does not apply to equation (4.7): ![]() exceeds
exceeds ![]() by
by ![]() . Hence, the differential dq is not a total
differential.
. Hence, the differential dq is not a total
differential.
A similar conclusion can also be obtained for another function of
process, the work of expansion
![]() (4.10)
(4.10)
Expressing this work as a function of two variables, pressure and
volume, l = f (p, v), the differential of this function
takes the following form:
![]() (4.11)
(4.11)
where x
= v and y = p.
Let us check whether the condition (4.6) is satisfied for this function.
Comparing (4.10) and (4.11), it is clear that M = p, N = 0 and, consequently,
![]()
![]()
whence
![]()
Thus,
the differential dl is not a total differential.
It should be borne in mind that if the path of a process proceeding from
state 1 to state 2 is strictly defined, the heat added to the
system in the process and the work of expansion done by the system in this
process will be determined unambiguously. So, if points 1 and 2 lie
on an isotherm, the heat added to the system in the process of isothermal
expansion, q12, and the work done by the
system in the course of isothermal expansion, l12, can be determined unambiguously if the system's
properties for the states 1 and 2 are given. It follows from this
that if the properties of state are given for point 1, the quantities q12 and l12 are single-valued
functions of the properties of state at point 2.
In this connection it is of interest to consider the concept of heat
capacity. In Chapter 1 heat capacity was defined as
![]()
Inasmuch as the heat q is a function of a process, in the general
form heat capacity is also a function of a process. It was, however, stipulated
above that in all instances it shall be specified what heat capacity or which
process is involved. Thus the heat capacity is
![]()
where x is a constant parameter. In
particular, if x = p,
then cx =
cp; if x = v, then cx = cv; etc. The heat capacities of
concrete processes already possess the properties of functions of state, i.e.
in a concrete process heat capacity is determined by the properties of state.
Consider relationship (4.4) applied to the case z = const. Then, dz
= 0 and, consequently,
![]()
whence
![]()
or,
which is the same,
![]() (4.12)
(4.12)
It is clear that if a certain quantity z is a function of two variables x and y, z = f (x, y), then,
on the same grounds, the quantity x can be considered as a function of
the variables y and z,
x = φ (y, z),
and the quantity y as a function of x and z, y = ψ (x, z).
Equation (4.12) relates unambiguously all possible derivatives of
these three functions. Equation (4.12) gives for p, v and T the
following relations:
![]() (4.12a)
(4.12a)
for p, T and s
![]() (4.12b)
(4.12b)
for h, u
and T
![]() (4.12c)
(4.12c)
etc.
Equation (4.3) yields one more useful relationship. Differentiating it
with respect to x, on
condition that a certain property of state ξ is constant we obtain:
![]() (4.13)
(4.13)
Equation (4.13) permits a relationship to be established between the
partial derivatives of one and the same quantities calculated, however, on the
basis of different constant parameters.