4.4 Heat capacities
Since
![]() and
and ![]()
we can write
![]() (4.44)
(4.44)
In particular, for the heat capacity at constant pressure,
![]() (4.45)
(4.45)
Since, as can be seen from Eq. (4.29), for an isobaric process (p = const)
![]()
we have
![]() (4.46)
(4.46)
Analogously, for the heat capacity at constant volume,
![]() (4.47)
(4.47)
With account taken of the fact that, as can be seen from Eq. (4.14), in an isochoric process (v = const)
![]()
we obtain from Eq. (4.47):
![]() (4.48)
(4.48)
The set of differential equations of thermodynamics permits the establishment of a number of important relations for heat capacities.
Differentiating
![]()
with respect to temperature at p = const, we obtain:
![]() (4.49)
(4.49)
To pass from the
partial derivative ![]() to the
derivative
to the
derivative ![]() make use of Eq. (4.13):
make use of Eq. (4.13):
![]() (4.50)
(4.50)
Using the relationship (4.25), we get:
![]() (4.51)
(4.51)
Substituting Eq. (4.51) into relation (4.49), we find that
![]() (4.52)
(4.52)
With the aid of Eq. (4.12a), the important equation (4.52) that relates the constant-pressure (isobaric) and constant-volume (isochoric) heat capacities, cp and cv, can also be presented in two forms:
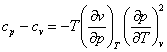 (4.53)
(4.53)
or
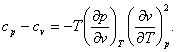 (4.54)
(4.54)
For an ideal gas,
![]() and
and ![]()
consequently,
![]()
Equation (4.45) can be rewritten in the following way:
![]()
Applying Maxwell's relation (4.20), we obtain:
![]() (4.55)
(4.55)
Analogously, for the constant-volume heat capacity Eq. (4.47) yields
![]()
whence, with account taken of
Maxwell's relation (4.21), we obtain an equation relating the constant-volume
heat capacity cv and the derivative ![]() ,
,
![]() (4.56)
(4.56)
The relation
expressing the dependence of cp on pressure, i.e. the
quantity ![]() is found by
differentiating Eq. (4.31) with respect to temperature at p = const:
is found by
differentiating Eq. (4.31) with respect to temperature at p = const:
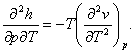
Since the sequence of differentiation does not effect a higher-order derivative,
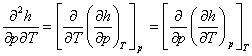
and, consequently,
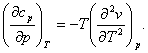 (4.57)
(4.57)
By analogy, from (4.25) we can derive the equation for the dependence of the constant-volume heat capacity, cv, on volume:
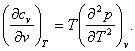 (4.58)
(4.58)
In conclusion, let us formulate one more essential thermodynamic equation, which relates the quantities cp and cv. Dividing (4.55) by (4.56), we get:
![]() (4.59)
(4.59)
Combining equations (4.59) with (4.52), we get the following two relations:
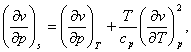 (4.60)
(4.60)
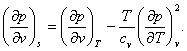 (4.61)
(4.61)